Published online Jan 14, 2014. doi: 10.3748/wjg.v20.i2.401
Revised: October 25, 2013
Accepted: November 28, 2013
Published online: January 14, 2014
The ultimate goals of treating chronic hepatitis B (CHB) is prevention of hepatocellular carcinoma (HCC) and hepatic decompensation. Since the advent of effective antiviral drugs that appeared during the past two decades, considerable advances have been made not only in controlling hepatitis B virus (HBV) infection, but also in preventing and reducing the incidence of liver cirrhosis and HCC. Furthermore, several recent studies have suggested the possibility of reducing the incidence of recurrent or new HCC in patients even after they have developed HCC. Currently, six medications are available for HBV treatment including, interferon and five nucleoside/nucleotide analogues. In this review, we will examine the antiviral drugs and the progresses that have been made with antiviral treatments in the field of CHB.
Core tip: Chronic hepatitis B virus (HBV) infection is one of the leading causes of death across the world due to its worldwide distribution and potential sequelae. Advances in knowledge in combination with the development of potent and effective antiviral therapy for chronic hepatitis B have led to decreased complications from the virus. Timely use of nucleotide/nucleosides may improve liver function and increase survival in patients with hepatic decompensation. Maintained suppression of HBV replication with antiviral therapy halts the progression of liver disease, may reverse liver fibrosis, and can reduce the development of cirrhosis and hepatocellular carcinoma.
- Citation: Marzio DHD, Hann HW. Then and now: The progress in hepatitis B treatment over the past 20 years. World J Gastroenterol 2014; 20(2): 401-413
- URL: https://www.wjgnet.com/1007-9327/full/v20/i2/401.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i2.401
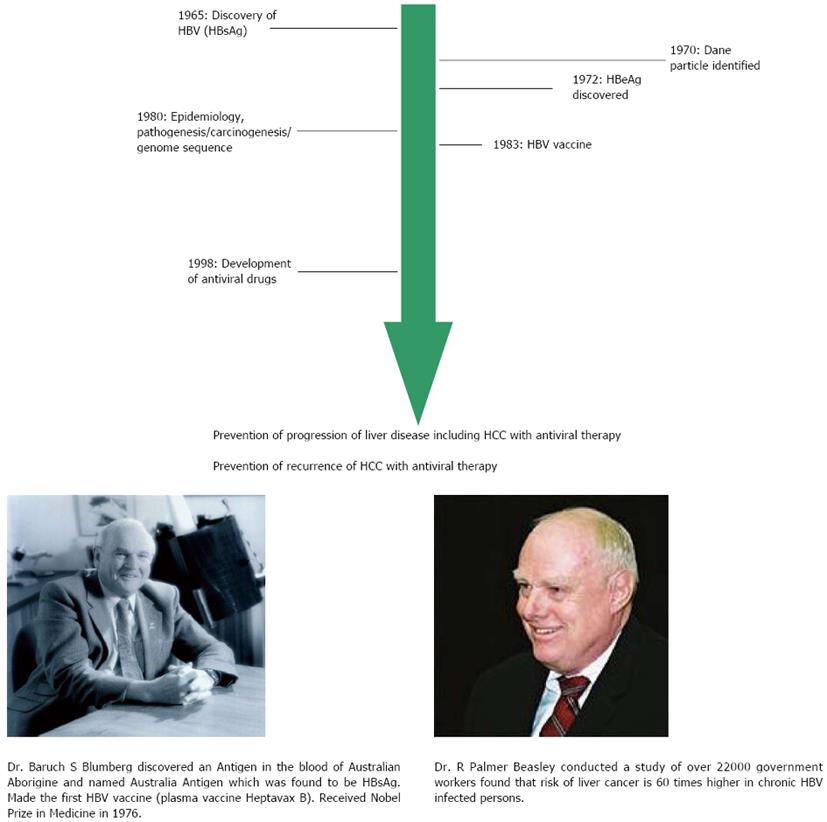
In the decades after World War II, clinical and epidemiological studies began to differentiate among various types of hepatitis[1]. However, it was the discovery of an antigen by Blumberg and his colleagues, now known as hepatitis B surface antigen (HBsAg), in the serum of an Australian Aborigine that reacted with the antibody (now known to be anti-HBs) in serum of a hemophiliac patient that provided the first clue[2]. Subsequent development of acute hepatitis in a technician in his laboratory provided the essential link to the illness. For his discovery and subsequent work on the disease progression related to hepatitis B virus (HBV), Blumberg received the Nobel Prize in Medicine 1976[3,4]. In 1970, Dane et al[5], identified the whole virus particle (Dane particle) using electron microscopy. In 1972, hepatitis B e antigen (HBeAg) was identified by Magnius et al[6]. By the early 1980’s the genome of the virus had been sequenced and the first vaccine (initiated by Millman et al and developed by Hilleman et al) were tested[7,8]. This plasma vaccine became available in 1983 and was rightly designated “The First Cancer Vaccine” by World Health Organization. The close link between HBV and hepatocellular carcinoma (HCC) was lucidly documented by Beasley et al[9] in their historical prospective study of 22707 men in Taiwan (Figure 1).
Since the discovery of the virus, our understanding and knowledge about the complexities of HBV have grown tremendously. Chronic HBV infection is one of the leading causes of death across the world due to its worldwide distribution and potential sequelae. People infected with the virus are at risk of developing hepatic decompensation, liver cirrhosis and HCC with 15% to 40% of individuals developing serious sequelae in their lifetime[9]. Despite the implementation of effective universal vaccination programs, over 300 million people are still chronically infected with HBV worldwide with 75% of infected individuals residing in the Asia-Pacific region[10,11].
Increased knowledge of the natural history of chronic hepatitis B (CHB) and clinical data demonstrating improved outcomes with medical interventions have led to publication of various treatment guidelines aimed at providing direction regarding initiation/on-treatment management of antiviral therapy and monitoring of outcome measures. These advances in knowledge in combination with the development of potent and effective antiviral therapy for CHB have led to decreased complications from the virus[12]. In this review, we will discuss the advances in the understanding of the natural history of CHB and the progress of anti-HBV treatment over the past two decades.
HBV belongs to a group of closely related DNA viruses termed Hepadnaviruses[13-15]. This family of viruses has a strong preference for infecting liver cells and has a similar life cycle in their hosts. The virus consists of a nucleocapsid and an outer envelope composed mainly of three HBsAgs, which play a central role in the diagnosis of HBV infection. The nucleocapsid contains hepatitis B core antigen (HBcAg), a DNA polymerase-reverse transcriptase, and the viral genome[16]. The genome consists of a partially double-stranded circular DNA molecule of about 3200 base pairs in length. The pre-surface 1/pre-surface 2 and surface genes code for the various HBsAgs. The protein encoded by the pre-core/core gene undergoes post-translational modification to yield HBeAg, which is a seromarker for high viral replication[16]. The viral DNA polymerase-reverse transcriptase is encoded by the polymerase gene and is of central importance for viral replication. Different from all known mammalian DNA viruses, hepadnaviruses replicate using a reverse transcription of an RNA intermediate[17-19]. Based on this unique replication cycle of HBV, antiviral therapeutic strategies have been mainly aimed at the reverse transcription of HBV RNA with nucleotide/nucleoside analogues[20].
The presence of HBV DNA in serum is the best indication of active viral replication. Antibody to HBsAg is produced in exposure to the envelope antigen and confers protective immunity. Antibody to HBcAg is detectable in all patients who have ever been exposed to HBV. However, unlike antibody to HBsAg, this antibody is not protective, but can be helpful in distinguishing acute from chronic infection if IgM antibody (anti-HBc IgM) is present. Antibody to HBeAg appears when the antigen has been cleared and the virus is no longer replicating or has reduced replication[12].
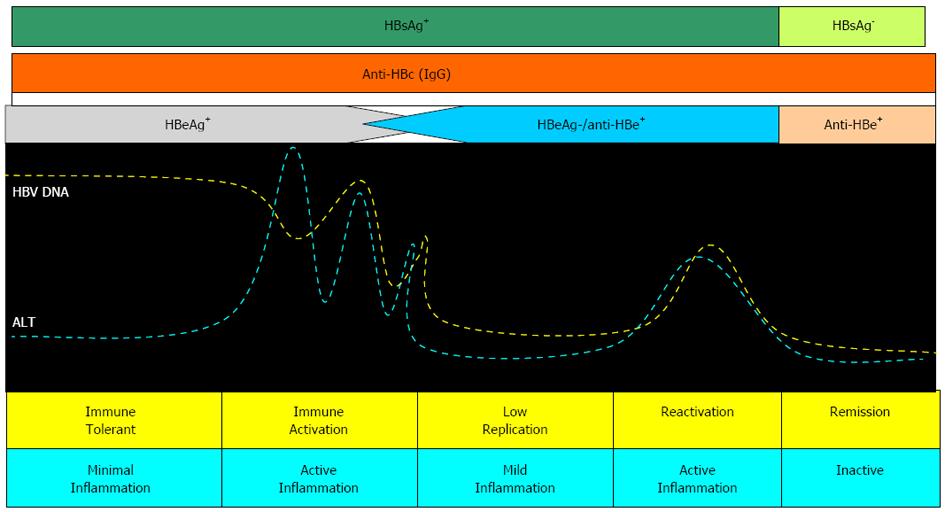
Liver injury in CHB is the result of the host’s immune responses against HBV; an HLA-class I antigen-restricted, cytotoxic T lymphocyte-mediated response against HBV antigens expressed on hepatocytes would result in apoptosis and necrosis of the hepatocytes[21]. Accordingly, CHB is a dynamic state of interactions among HBV, the patient’s hepatocytes and the immune system. Based on these interactions, the natural course of CHB can be divided into different changing phases, although not all patients go through all of the phases (Figure 2)[22].
The first phase is the “immune tolerant phase” which consists of HBeAg seropositivity, high viral loads, but with a normal serum alanine aminotransferase (ALT) and near-normal liver histology. Adult-acquired chronic HBV infection usually has a very short “immune tolerant phase”. In contrast, the perinatally or early childhood-acquired chronic HBV infection has a long “immune tolerant phase”[22-24]. The “immune clearance phase” usually develops during adolescence or adulthood. This phase is characterized by positive HBeAg, high serum HBV levels and increased ALT levels, sometimes complicated by hepatic decompensation[21]. These events may lead to progression to fibrosis or development of cirrhosis in some patients during the HBeAg-positive phase, but may also result in a declining serum HBV DNA level and may eventually lead to HBV DNA seroclearance and HBeAg seroconversion to its antibody (anti-HBe) in most patients. A 3-year clinical study in patients with chronic hepatitis B, or patients in the “immune clearance phase”, showed that cirrhosis developed at an estimated annual incidence of 2.1%, being higher in those seropositive for HBeAg at entry (2.4%/year)[25]. The estimated annual incidence of spontaneous HBeAg seroconversion was reported to be 2%-15%, depending on factors such as age, ALT level and HBV genotype[26,27]. Following HBeAg seroconversion, most of the patients enter an “inactive phase” with sustained normal serum ALT, low serum HBV DNA and no or minimal necro-inflammatory histological changes, although some of them may have already developed advanced fibrosis or cirrhosis[27-29]. Spontaneous HBsAg seroclearance may occur several years after HBeAg seroconversion at an incidence of 0.7%-2.4% per year depending on age at time of infection[29].
As early as the 1970’s, chronic infection with the HBV was associated with the development of HCC. A powerful substantiation of the association between HBV infection and HCC was the results of a prospective cohort study reported by Beasley et al[9] in 1981. These investigators followed more than 22000 male municipal workers in Taiwan and found that those who were seropositive for HBsAg had rates of HCC that were significantly greater than were the rates in uninfected controls. They calculated the relative risk for HCC among those who were HBV-infected to be 63 compared to uninfected controls. More recent cohort studies have confirmed the high risk of HCC in HBsAg-positive individuals as originally identified in the Beasley study. An example is the Haimen City cohort that included about 11000 HBsAg-positive subjects followed over a mean period of 8 years[30]. The mechanism by which HBV infection causes HCC is not completely understood. Evolution to HCC may be the direct effect of the virus itself, or it may be an indirect effect, through the process of the inflammation, regeneration and fibrosis associated with cirrhosis due to the HBV infection[31,32]. HBV DNA has been shown to become integrated within the chromosomes of infected hepatocytes, the integration of viral genetic material occurring in a critical location within the cellular genome. The hepatitis B x gene (HBx) product has been implicated in causing HCC because it is a transcriptional activator of various cellular genes associated with growth control[32,33]. The HBx gene expression is also associated with activation of the Ras-Raf-MAP kinase pathway, an important cellular pathway that has been implicated in hepatocarcinogenesis.
HBsAg seroclearance usually confers protection against HCC but may still carry a risk for HCC although at a very low rate and usually in patients in whom cirrhosis or superinfection with other viruses had already developed before HBsAg seroclearance[34,35]. Studies further indicate that serum HBV DNA level is associated with cirrhosis and HCC development in a dose-dependent manner starting from serum HBV DNA level[34-38]. These findings suggest that HBV replication, with subsequent immune-mediated liver injuries, is the primary driving force for liver disease progression. It has also been identified that patients of Asian background are at higher risk for HCC because they are more likely to have been infected early in life and carcinogenic processes could have taken place earlier[37]. This may explain why some patients even with well suppressed viral replication still develop HCC.
As stated earlier, HBV infection is common and clinically consequential worldwide. In endemic countries, an estimated 50 million new cases are diagnosed annually. In Asia, HBV is the leading cause of chronic hepatitis, cirrhosis, and HCC[37]. The HBV carrier rates in Asia have been reported to be as high as 20% in the male population of Guangxi Province, China[38]. A recent study, conducted in China, showed that HBV carrier rates have fallen to 7.2% in regions where hepatitis B vaccination programs had been implemented[39]. In South Korea, the HBV carrier rates ranged from 5.0% to 8.6% in the 1970s and 1980s and have subsequently declined to 3.7%-5.7% as a result of national vaccination programs[40,41]. In other parts of Asia, the HBV infection rates remain high, particularly in countries in which vaccine programs have not yet been implemented. Notably, the HBsAg rates among Asians residing in the United States are similar to rates reported in their countries of origin, especially in first-generation immigrants to the United States[42-45]. Therefore, the disease burden from HBV, including mortality from liver disease progression and development of HCC, remains a major health problem among Asian Americans with CHB.
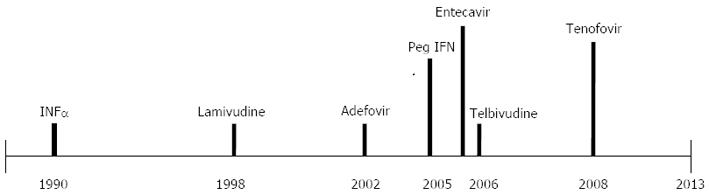
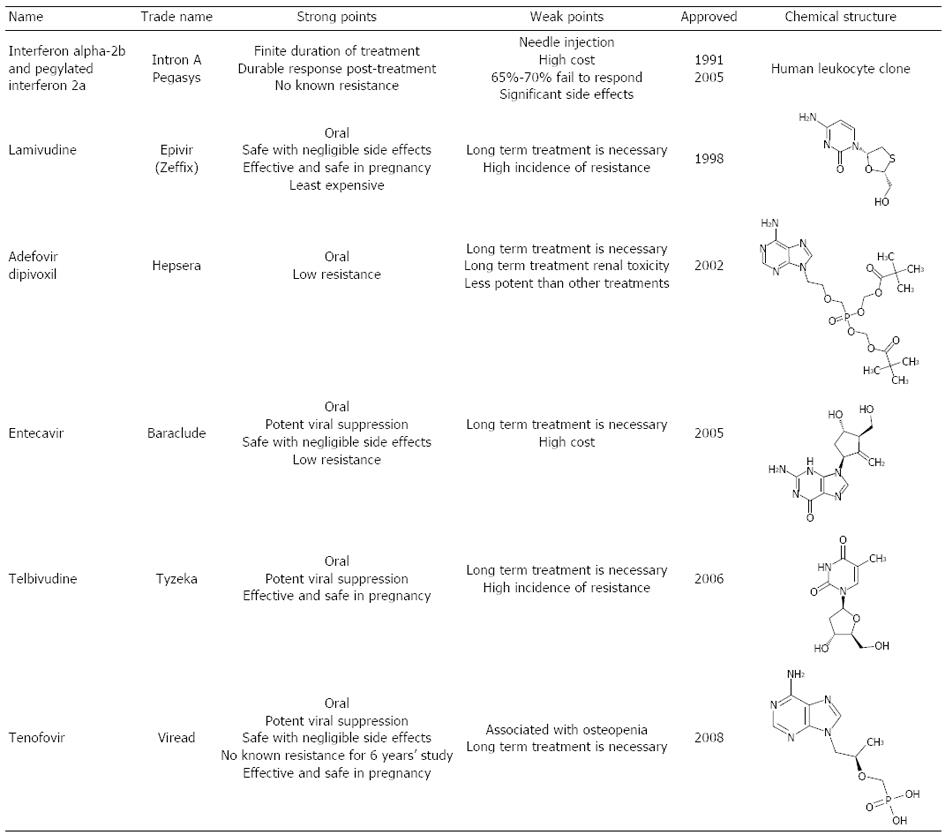
Currently, six treatments are approved for hepatitis B, including interferon (IFN) (two formulations: IFN and PEG-IFN) and five nucleotide/nucleoside analogues (lamivudine, adefovir, entecavir, telbivudine and tenofovir) (Figures 3 and 4)[36,46]. The aim of hepatitis B treatment is to achieve sustained viral suppression of HBV replication. With viral suppression, the ultimate goal would be prevention of cirrhosis and HCC. Response to treatment is judged based on decrease in serum HBV DNA level, loss of HBeAg with or without seroconversion to antiHBe, loss of hepatitis B surface antigen (HBsAg) with or without seroconversion to HBs antibody, normalization of serum ALT levels, and a decrease in hepatic inflammation on liver biopsy[46,47].
The importance of monitoring individuals with low-serum HBV DNA and normal ALT levels regardless of their HBeAg status has also become recognized in recent years. Serial ALT and HBV DNA monitoring every 3 mo for 1 year after the initial diagnosis and 6-12 mo thereafter is usually recommended to detect intermittent flares of hepatitis B[37,48]. This regimen is also useful to differentiate chronic active HBeAg negative hepatitis from inactive carriers in recently diagnosed HBV carriers. Treatment should be initiated regardless of the level of viremia if active inflammation is also detected on liver biopsy[37,47,49]. In the case of HBeAg negative CHB, treatment is continued indefinitely until HBsAg becomes undetectable. Nonetheless, close follow-up is important, and prompt retreatment is necessary if elevation of HBV DNA and ALT levels are observed[37]. Therefore, achieving maximum viral suppression without the development of antiviral drug resistance, reducing progression to cirrhosis and decreasing the risk of developing HCC are the primary treatment endpoints.
In 1991, conventional IFNα-2a was the first successful treatment approved for CHB with widespread use. Its major mechanism of action is immune modulation, although there is also a weak anti-viral effect[50]. Peg IFNα-2a replaced standard IFN in 2005 due to improved pharmacokinetic properties and a less demanding injection schedule with comparable efficacy. Long-term follow-up of patients treated with conventional IFN therapy showed that responders had a decreased incidence of hepatic decompensation and HCC, and improved overall survival compared with non responders[51-53]. Forty-eight weeks of therapy with Peg IFN results in a 27% rate of HBeAg seroconversion and 25% rate of loss of HBV DNA. Six months after discontinuation of therapy, the HBeAg seroconversion rates increased to 32%. Loss of HBsAg with the appearance of anti-HBs occurred in 4%-6% of patients after 1 year of treatment and 6 mo of post treatment follow-up[51,54,55]. Even after discontinuation of IFN therapy, 12%-65% of patients lost HBsAg within 5 years of HBeAg loss. This results in the highest rate of off-treatment sustained response after 1 year of therapy[53,54]. Achieving early virological response, defined as > 2 log10 drop in serum HBV DNA or suppression to levels below 105 copies/mL in the first 2 wk of therapy, is associated with induction of long-term remission after stopping therapy[56,57].
Despite the fixed duration of Peg IFN therapy and the lack of antiviral drug resistance compared with oral agents, the use of Peg IFN only accounts for no more than 10% of all prescriptions for the treatment of CHB in the United States[54]. This low rate can be explained by the drug’s substantial side effect profile and the need for administration by injection.
Lamivudine (LAM) was the first nucleoside analogue reverse transcriptase inhibitor that was approved for use by the United States Food and Drug Administration (FDA) in 1998. Although it is not as used as commonly today due to the presence of better oral agents with higher genetic barrier to resistance; it played a major role in the transition CHB treatment and allowed reduction in cirrhosis and risk of HCC to be achieved with some success.
One-year therapy with lamivudine is associated with 16%-18% rate of HBeAg seroconversion; the HBeAg seroconversion rate increases to 50% with 5 years of therapy[58-60]. LAM therapy also results in 60%-70% HBV DNA suppression in HBeAg-negative CHB after 1 year of therapy[56]. The durability of response is lower than the interferon therapy regardless of the HBeAg status and has been reported to range between 50% and 80% for HBeAg-positive CHB and 20%-25% for HBeAg-negative CHB patients.
Treatment of HBV with LAM has been shown to slow the rate of development of fibrosis, as well as decrease of HCC incidence[60-62]. Use of LAM is associated with a significant risk of development of resistance with prolonged use. Five years of therapy can lead to 65%-70% rate of resistance[59]. However, thorough review of studies investigating the LAM resistance during CHB treatment revealed a diverse range of methodologies to assess resistance[63]. Studies that use purely genotypic methods report resistance rates at 1 year ranging from 14% to 32%. However, these may overestimate clinically relevant resistance. Studies that use virologic resistance report lower one year resistance rates, ranging from 6.4% to 15.4% and may provide more relevant measure of resistance. When comparing resistance rates with antiviral drugs in CHB, it is important to consider the methodology and definition of resistance used[63]. In addition, baseline HBV DNA was closely related to the resistance rate[64] and HBV DNA level at 6 mo on LAM therapy was an important predictor for LAM resistance at one year and later[65].
The largest and most compelling study suggesting that antiviral treatment might decrease the risk of HCC was a randomized, controlled trial of LAM vs placebo in patients with advanced chronic hepatitis B and high serum levels of HBV DNA[60]. The primary outcome of the study was progression of liver disease, including an increase in Child-Pugh score, bleeding from esophageal varices and the development of HCC. The study was halted early because of a distinct benefit for the group on LAM treatment compared to the placebo group. Instead of continuing the study for intended 5 years, all received LAM at the end of 3 years on trial. In this study, at year 3, the rate of HCC was 3.9% among LAM recipients vs 7.4% among placebo recipients (P = 0.047). Other retrospective studies observed similar results[61,66].
Adefovir dipivoxil (ADV) was the first nucleotide analogue approved in United States in 2002 for the treatment of CHB. The arrival of this agent provided new insights into the treatment of CHB. ADV did not only have increased antiviral potency but also had an intrinsic stereoscopic structure which was an important factor against the emergence of viral resistance.
One year of therapy with ADV leads to a 12% rate of HBeAg seroconversion and 53% rate of histological improvement in HBeAg-positive patients[67]. Once HBeAg seroconversion occurs, it is sustained in 91% of patients[68]. Like LAM, HBeAg-negative patients require therapy indefinitely with ADV, and resistance is also a problem with prolonged ADV use. Persistence of viremia after 48 wk of therapy is linked to development of resistance[69]. Resistance rates of 0%, 3%, 18% and 29% have been reported at 1, 2, 4 and 5 years of therapy[70]. These high resistance rates and its potential renal toxicity have lead to declining use of ADV in light of newer therapeutic agents. Furthermore, ADV was highly effective for LAM resistant HBV[71,72].
During the period between 1998 and 2004, LAM and ADV for treatment naive CHB and ADV for LAM-resistant CHB were the main treatment strategies available for CHB. In 2005, entecavir (ETV), a nucleoside analogue, entered the arena when it was approved in United States. It is a potent inhibitor of HBV polymerase at a dose of 0.5 mg daily resulting in 6.98 log10 copies/mL decrease in HBV DNA levels compared to a 5.4 log10 copies/mL reduction with LAM[73]. In clinical studies, patients who received ETV for 52 wk achieved superior virological response, with HBV DNA < 400 copies/mL (67% vs 36%), histological improvement (72% vs 62%) and normalization of ALT (78% vs 70%) compared with those who received LAM[73]. However, there was no difference between ETV and LAM in achieving HBeAg seroconversion (21% vs 18%). ETV is also superior to LAM in HBeAg-negative patients, but requires indefinite treatment to maintain viral suppression to prevent relapse[74-76]. ETV demonstrates better virological suppression (91% vs 73%) and improved histology (70% vs 61%). In addition, analysis of two studies of patients who received continuous ETV for up to 5 years revealed that 94% of patients continue to have HBV DNA < 300 copies/mL at 5 years[76].
In long term studies, up to 96% of patients (mainly HBeAg-positive CHB) had histological improvement and 88% of the patients had improvement in fibrosis score after 6 years of ETV therapy; this holds true even in patients with cirrhosis[77]. ETV has also been shown to be superior to ADV in achieving rapid viral suppression within 2 wk of therapy. Even though a 48-wk therapy with ETV compared with ADV was associated with a higher rate of HBV clearance (58% vs 19%) and ALT normalization (76% vs 63%), there was no difference in the rate of HBeAg loss (18% vs 22%) and HBeAg seroconversion (15% vs 22%)[78].
One of the most important differences from LAM and ADV is that, ETV has a high genetic barrier with a very low incidence of resistance. The cumulative incidence rate of resistance after 6 years of therapy in nucleoside-naïve patients remains low at 1.2%[79,80]. The low resistance rate is related to both profound viral suppression and the requirement of at least 3 sites of genetic mutations to confer resistance. However, the chance for ETV resistance is much higher in patients who already developed LAM resistance[80]. ETV therapy has also been associated with HBsAg loss, improvement of liver histology, decreased risk of HCC and very low to undetectable HBV DNA levels[81,82]. The ability to decrease the incidence of HCC in patients with CHB has been the most exciting attributes of antiviral therapy. Recent report from Hosaka et al[83] compared the incidence of HCC in 472 ETV-treated patients and 1143 non-treated HBV patients. The drug mutation resistance was 0.8% (4/472) in the ETV group. The cumulative HCC incidence rates at 5 years were 3.7% and 13.7% for the ETV and control groups, respectively (P < 0.001). The treatment effect was found to be greater in patients at higher risk of HCC.
While newer treatments for LAM-resistant disease were still under investigation, telbivudine (TLV), another nucleoside analogue was approved by the FDA in 2006 for treatment of chronic CHB. In HBeAg-positive CHB patients, the rate of HBeAg seroconversion with TLV therapy was 22% and 30% at 1 and 2 years respectively. Viral suppression was limited to HBV DNA levels of < 300 copies/mL after 1 and 2 years of therapy in 60% and 56% of HBeAg-positive patients[84]. In HBeAg-negative CHB patients, HBV suppression was noted in 88% and 82% of patients at 1 and 2 years of therapy, respectively[84,85]. Resistance to TLV has been reported to be 21.6% in HBeAg-positive patients and 8.6% in HBeAg-negative patients after 1 and 2 years of therapy respectively. Although TLV barrier to antiviral resistance is higher then LAM, is not recommended as a first-line agent[37,48,51]. Although this treatment is not used often due to its high rate of resistence it is effective and safe for the prevention of mother-to-child transmission of HBV from chronically infected mothers with a high degree of infectivity late in pregnancy.
Analysis of the baseline characteristics of the patients enrolled in the GLOBE trial revealed important predictive factors of response to therapy. The strongest predictors of achieving a good response to TLV therapy in HBeAg-positive patients were serum HBV DNA < 9 log10 copies/mL, or ALT ≥ 2 times the ULN at baseline with undetectable serum HBV DNA at week 24 of therapy[86]. Extensive review of TLV for treatment of hepatitis B indicated that there was a specific group of patients who are likely to achieve good therapeutic response with TLV. Patients with low baseline HBV DNA who could achieve negative HBV DNA at week 24 had the best outcome with TLV[87]. With this data, LAM and TLV can be used in countries where cost is a major concern by selecting patients with favorable baseline HBV DNA and ALT levels. Another important aspect of TLV is its renoprotective effect as recently reported by Gane et al[88]. In approximately 2500 patients treated with TLV, there was a trend towards in increased GFR in both compensated and decompensated CHB. The mechanism of this renoprotective effect by TLV is unknown.
Rescue therapy for patients with viral resistance to the nucleoside analogues was the usage of adefovir until 2008 when, tenofovir disoproxil fumarate (TDF), the second nucleotide analogue was approved for the treatment of CHB. It is structurally related, but more potent than ADV. Forty-eight weeks of TDF compared with ADV therapy in HBeAg-positive CHB resulted in more patients achieving viral suppression defined as < 400 copies/mL (76% vs 13%), normalization of ALT (68% vs 54%), histological improvement (67% vs 12%) and HBsAg loss (3.2% vs 0%)[89]. Data from the TDF trials revealed an excellent durability of response, with a viral suppression (HBV DNA < 400 copies/mL) of 99% and 100% in HBeAg-negative and HBeAg-positive CHB respectively after 4 years of therapy[90,91]. Sub-analysis of the Asian subset of 145 patients revealed similar efficacy (97%) in achieving viral suppression defined as HBV PCR < 400 copies/mL[92]. Four years of TDF therapy has led to HBeAg loss in 41% of patients and HBeAg seroconversion in 29%[91]. TDF is also superior to ADV in achieving increased viral suppression (93% vs 63%), an improved inflammatory score and viral suppression (71% vs 49%) in a phase III study of HBeAg-negative patients[89]. However, besides profound viral suppression, the most impressive characteristic of TDF is that no resistance has been detected to date with 5 years of follow up[92,93]. Due to these excellent features, TDF is recommended as first line agent for treatment-naïve CHB patients. Furthermore, treatment with TDF for 5 years showed regression of cirrhosis in 74% of those who showed cirrhosis at baseline[94].
Emtricitabine is a nucleoside analogue structurally similar to LAM. Emtricitabine was approved by the FDA since 2003 for treatment of HIV infection and is not approved by the FDA for CHB. It is currently being studied as an add-on to TDF therapy in the form of Truvada (tenofovir 300 mg/emtricitabine 200 mg). Like lamivudine, its use as monotherapy for treatment of CHB is limited by its intermediate genetic barrier to resistance. Two years of emtricitabine therapy is associated with 13% risk of development of resistance[95].
A randomized trial in ADV-experienced patients showed equal efficacy in viral suppression to < 400 copies/mL between tenofovir and Truvada at 24 wk of therapy[96]. After 24 wk in the randomized arm, patients were switched to open label Truvda if they had detectable HBV DNA defined as > 400 copies/mL. Eighty one per cent of patients in each treatment arm achieved serum HBV DNA < 400 copies/mL at the end of week 48 according to intention-to-treat analysis[96]. The presence of baseline ADV resistance or LAM resistance did not impact the efficacy of TDF nor Truvada. Both TDF and Truvada were equivalent through week 168 of therapy in achieving viral suppression at a rate of 82%, independent of pre-existing ADV or LAM-resistant mutations[97].
With the advent of antiviral therapy, it is now possible to reduce inflammation, regress cirrhosis and reduce the incidence of HCC in patients with CHB. The incidence of HCC recurrence after resection of HBV-related HCC is high. Newer data has shown that there is a role for antiviral therapy for those who have already developed HCC. Since 2005, there have been retrospective studies, small and large in numbers that showed improvement of survival in patients who received concomitant antiviral therapy after curative liver resection and local tumor ablation[98-102]. Treatment with nucleoside/nucleotide analogues may prevent de novo primary tumors and further progression of liver disease, thereby decreasing recurrent HCC. Recent large cohort studies further confirmed the benefit of antiviral therapy in this group of patients with decrease in mortality with the antiviral treatment[103,104]. The longest survivors of those who benefited from antiviral therapy following the existing tumor ablation have reached over 12 years (Hann et al, personal communication). This novel treatment strategy may offer a significant alternative to liver transplantation to relieve the current graft shortage.
As noted above, the development of anti-viral resistance is a barrier in achieving successful therapy in CHB. In large retrospective review of 11000 CHB patients on nucleoside/nucleotide therapy, mean adherence rate to therapy was 87.8% with 1-year persistence of 81%. Although adherence to CHB therapy is high, new and younger age patients tend to be less compliant[105]. In a study of 148 CHB patients on nucleoside/nucleotide therapy with mean follow-up of 3 years, 39 patients had at least one virologic rebound with 38% having no genotypic resistance and 10 patients with further HBV DNA decline while continued on current re-treatment[106]. Medication non-adherence is a common cause of intermittent virologic rebound and should be addressed before changing therapy. In a study of 84 patients treated with LAM, ADV, or ETV who stopped therapy after reaching defined endpoints, 42% of HBeAg-positive and 47% HBeAg negative patients had virologic relapse with HBV DNA more than 1000 copies/mL at a mean of 4.3 mo[107].
Concerns about the possible lose of bone mineral density (BMD) and has been raised from the results of clinical studies on CHB treatment[108-111]. BMD loss has been reported in chronic liver disease. However, accelerated BMD loss has been reported in patients specifically on TDF[108]. This BMD loss has raised concerns regarding the long term safety of TDF. BMD should be monitored in patients on TDF with bone density scans and factors that also contribute to bone loss should be given consideration when selecting a treatment option for CHB[108,109]. Like BMD, renal function is frequently impaired in patients with compensated CHB. These oral antiviral agents are all primarily eliminated unchanged through renal route. Therefore, inpatients with renal insufficiency, dose reduction and/or increased dose intervals are recommended. Renal impairment is frequent after long-term treatment with adefovir[110]. Similarly, a decrease of eGFR has been observed in retrospective cohorts of CHB patients during long-term tenofovir or entecavir-treated[111].
Although a vaccine has been available for hepatitis B since 1982, this chronic infection is still far from eradicated across the world. Timely use of nucleotide/nucleosides may improve liver function and increase survival in patients with hepatic decompensation. Maintained suppression of HBV replication with antiviral therapy halt the progression of liver disease, may reverse liver fibrosis, and can reduce the development of cirrhosis and HCC. Due to the availability of effective and potent treatment options for HBV, there has been a decrease in the proportion of annual liver transplants performed for this indication[112]. However, one must remember that this can only achieved with an excellent compliance on the part of patients, early detection of drug resistance and correct choice of medications. Nonetheless, current therapies may not always prevent all adverse sequela. HCC must be monitored using ultrasound and α-fetoprotein assays to improve outcomes by increasing early detection and the chance of curative treatment. Developing safe and affordable agents as well as management strategies to improve sustained HBV suppression should be the ultimate goal in the treatment of chronic HBV infection.
P- Reviewers: Fernandez-Rodriguez CM, Gilles G S- Editor: Song XX L- Editor: A E- Editor: Zhang DN
| 1. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [PubMed] [Cited in This Article: ] |
| 2. | Blumberg BS, Alter HJ, Visnich S. A “new” antigen in leukemia sera. JAMA. 1965;191:541-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 885] [Cited by in F6Publishing: 918] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [PubMed] [Cited in This Article: ] |
| 4. | Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977;197:17-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 127] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1:695-698. [PubMed] [Cited in This Article: ] |
| 6. | Magnius LO, Espmark JA. New specificities in Australia antigen positive sera distinct from the Le Bouvier determinants. J Immunol. 1972;109:1017-1021. [PubMed] [Cited in This Article: ] |
| 7. | Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 723] [Cited by in F6Publishing: 769] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 8. | Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, Sadovsky R, Morrison JM, Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303:833-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 791] [Cited by in F6Publishing: 654] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1838] [Cited by in F6Publishing: 1721] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 10. | Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 350] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [PubMed] [Cited in This Article: ] |
| 13. | Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533-4537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 483] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Mason WS, Seal G, Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980;36:829-836. [PubMed] [Cited in This Article: ] |
| 15. | Marion PL, Oshiro LS, Regnery DC, Scullard GH, Robinson WS. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci USA. 1980;77:2941-2945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 269] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Halpern MS, England JM, Deery DT, Petcu DJ, Mason WS, Molnar-Kimber KL. Viral nucleic acid synthesis and antigen accumulation in pancreas and kidney of Pekin ducks infected with duck hepatitis B virus. Proc Natl Acad Sci USA. 1983;80:4865-4869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Barker LF, Maynard JE, Purcell RH, Hoofnagle JH, Berquist KR, London WT, Gerety RJ, Krushak DH. Hepatitis B virus infection in chimpanzees: titration of subtypes. J Infect Dis. 1975;132:451-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 103] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Summers J, Mason WS. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403-415. [PubMed] [Cited in This Article: ] |
| 19. | Klingmüller U, Schaller H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol. 1993;67:7414-7422. [PubMed] [Cited in This Article: ] |
| 20. | Pollack JR, Ganem D. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J Virol. 1994;68:5579-5587. [PubMed] [Cited in This Article: ] |
| 21. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in F6Publishing: 1171] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 22. | Chu CM, Karayiannis P, Fowler MJ, Monjardino J, Liaw YF, Thomas HC. Natural history of chronic hepatitis B virus infection in Taiwan: studies of hepatitis B virus DNA in serum. Hepatology. 1985;5:431-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 273] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173-S181. [PubMed] [Cited in This Article: ] |
| 24. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [PubMed] [Cited in This Article: ] |
| 25. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 428] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol. 2005;43:411-417. [PubMed] [Cited in This Article: ] |
| 28. | Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-834. [PubMed] [Cited in This Article: ] |
| 29. | Zacharakis GH, Koskinas J, Kotsiou S, Papoutselis M, Tzara F, Vafeiadis N, Archimandritis AJ, Papoutselis K. Natural history of chronic HBV infection: a cohort study with up to 12 years follow-up in North Greece (part of the Interreg I-II/EC-project). J Med Virol. 2005;77:173-179. [PubMed] [Cited in This Article: ] |
| 30. | Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT. Eight-year follow-up of the 90,000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev. 2002;11:369-376. [PubMed] [Cited in This Article: ] |
| 31. | Blum HE, Moradpour D. Viral pathogenesis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17 Suppl 3:S413-S420. [PubMed] [Cited in This Article: ] |
| 32. | Hann HW, Feitelson M. Hepatocellular Carcinoma Associated with Hepatitis B Virus. Hepatocellular Carcinoma: Diagnosis and Treatment. 2nd ed. Totowa: Humana Press 2010; 235-257. [Cited in This Article: ] |
| 33. | Muroyama R, Kato N, Yoshida H, Otsuka M, Moriyama M, Wang Y, Shao RX, Dharel N, Tanaka Y, Ohta M. Nucleotide change of codon 38 in the X gene of hepatitis B virus genotype C is associated with an increased risk of hepatocellular carcinoma. J Hepatol. 2006;45:805-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] [Cited in This Article: ] |
| 35. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 924] [Cited by in F6Publishing: 870] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 36. | Hann HW. Active antiviral therapy for chronic hepatitis B and hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2008;54:19-30. [PubMed] [Cited in This Article: ] |
| 37. | Tong MJ, Pan CQ, Hann HW, Kowdley KV, Han SH, Min AD, Leduc TS. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci. 2011;56:3143-3162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Yeh FS, Yu MC, Mo CC, Luo S, Tong MJ, Henderson BE. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989;49:2506-2509. [PubMed] [Cited in This Article: ] |
| 39. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550-6557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 633] [Cited by in F6Publishing: 678] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 40. | Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol. 2009;15 Suppl 6:S13-S24. [PubMed] [Cited in This Article: ] |
| 41. | Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010;53:20-28. [PubMed] [Cited in This Article: ] |
| 42. | Hann HW, Kim CY, London WT, Whitford P, Blumberg BS. Hepatitis B virus and primary hepatocellular carcinoma: family studies in Korea. Int J Cancer. 1982;30:47-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 351] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 44. | Tong MJ, Hwang SJ. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am. 1994;23:523-536. [PubMed] [Cited in This Article: ] |
| 45. | Hann HW, Hann RS, Maddrey WC. Hepatitis B virus infection in 6,130 unvaccinated Korean-Americans surveyed between 1988 and 1990. Am J Gastroenterol. 2007;102:767-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Ayoub WS, Keeffe EB. Review article: current antiviral therapy of chronic hepatitis B. Aliment Pharmacol Ther. 2011;34:1145-1158. [PubMed] [Cited in This Article: ] |
| 47. | Yuen MF, Lai CL. Treatment of chronic hepatitis B: Evolution over two decades. J Gastroenterol Hepatol. 2011;26 Suppl 1:138-143. [PubMed] [Cited in This Article: ] |
| 48. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-141; quiz 1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 49. | Wiegand J, van Bömmel F, Berg T. Management of chronic hepatitis B: status and challenges beyond treatment guidelines. Semin Liver Dis. 2010;30:361-377. [PubMed] [Cited in This Article: ] |
| 50. | Dianzani F. Biological basis for the clinical use of interferon. Gut. 1993;34:S74-S76. [PubMed] [Cited in This Article: ] |
| 51. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2125] [Cited by in F6Publishing: 2114] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 52. | Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA, Niesters HG, Zondervan P, Hansen B, Schalm SW. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 918] [Cited by in F6Publishing: 858] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 53. | Papatheodoridis GV, Manesis E, Hadziyannis SJ. The long-term outcome of interferon-alpha treated and untreated patients with HBeAg-negative chronic hepatitis B. J Hepatol. 2001;34:306-313. [PubMed] [Cited in This Article: ] |
| 54. | Zoulim F, Perrillo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48 Suppl 1:S2-19. [PubMed] [Cited in This Article: ] |
| 55. | Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002-2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 56. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1188] [Cited by in F6Publishing: 1098] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 57. | Lau DT, Everhart J, Kleiner DE, Park Y, Vergalla J, Schmid P, Hoofnagle JH. Long-term follow-up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology. 1997;113:1660-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 224] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 58. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [PubMed] [Cited in This Article: ] |
| 59. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 584] [Cited by in F6Publishing: 638] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 60. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1739] [Cited by in F6Publishing: 1648] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 61. | Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 549] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 63. | Hann HW, Gregory VL, Dixon JS, Barker KF. A review of the one-year incidence of resistance to lamivudine in the treatment of chronic hepatitis B: Lamivudine resistance. Hepatol Int. 2008;2:440-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 64. | Chae HB, Hann HW. Baseline HBV DNA level is the most important factor associated with virologic breakthrough in chronic hepatitis B treated with lamivudine. World J Gastroenterol. 2007;13:4085-4090. [PubMed] [Cited in This Article: ] |
| 65. | Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34:785-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 319] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 66. | Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1060] [Cited by in F6Publishing: 1107] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 68. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807. [PubMed] [Cited in This Article: ] |
| 69. | Locarnini S, Qi W, Arterburn S. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil (ADV) therapy for patients with chronic hepatitis B (CHB). Hepatology. 2005;42:17A. [Cited in This Article: ] |
| 70. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 639] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 71. | Peters MG, Hann Hw Hw, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df Df. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 469] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 72. | Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 73. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1107] [Cited by in F6Publishing: 1032] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 74. | Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, Han S, Kaymakoglu S, Tamez R, Yang J. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50:289-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 915] [Cited by in F6Publishing: 865] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 76. | Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 478] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 77. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 717] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 78. | Leung N, Peng CY, Hann HW, Sollano J, Lao-Tan J, Hsu CW, Lesmana L, Yuen MF, Jeffers L, Sherman M. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of entecavir versus adefovir. Hepatology. 2009;49:72-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 79. | Tenney DJ, Pokornowsky KA, Rose RE, Baldick CJ, Eggers BJ, Fang J, Wichroski MJ, Diva UA, Xu D, Wilber RB. Entecavir maintains a high genetic barrier to HBV resistance through 6 years in naive patients. J Hepatol. 2009;50:S10. [Cited in This Article: ] |
| 80. | Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 615] [Cited by in F6Publishing: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 81. | Chu CM, Liaw YF. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther. 2010;15:133-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 82. | Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123:1084-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 83. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 84. | Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-2588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 652] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 85. | Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 86. | Chan HL, Heathcote EJ, Marcellin P, Lai CL, Cho M, Moon YM, Chao YC, Myers RP, Minuk GY, Jeffers L. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med. 2007;147:745-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 87. | Hann HW. Telbivudine: an effective anti-HBV drug for chronic hepatitis B patients with early on-treatment responses. Expert Opin Pharmacother. 2010;11:2243-2249. [PubMed] [Cited in This Article: ] |
| 88. | Gane E, Chan HLY, Deray G, Piratvisuth T, Zeuzem S, Jia J, Ren H, Uddin A, Bosset B, Dong Y. Renal function improves with long-term telbivudine therapy in patients with chronic hepatitis B Analysis of the Clinical data Base. 14th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV. 2012;Jul 19-21; Washington, DC. [Cited in This Article: ] |
| 89. | Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442-2455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 890] [Cited by in F6Publishing: 851] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 90. | Marcellin P, Buti M, Krastev Z, Gurel S, Di Bisceglie AM, Odin JA, Dusheiko GM, Heathcote EJ, Borroto-Esoda K, Coombs DH. Continued efficacy and safety through 4 years of tenofovir disoproxil fumarate (TDF) treatment in HBeAg negative patients with chronic hepatitis B (study 102): preliminary analysis. Hepatology. 2010;52:555A. [Cited in This Article: ] |
| 91. | Heathcote EJ, Gane EJ, De Man R, Lee SS, Flisiak R, Manns MP. Long term (4 years) efficacy and safety of tenofovir disoproxil fumarate (TDF) treatment in HBeAg-positive patients (HBeAg) with chronic hepatitis B (Study 103): preliminary analysis. Hepatology. 2010;52:556A. [Cited in This Article: ] |
| 92. | Gane E, Lee SL, Heathcote EJ, Sievert W, Trinh H. Four years efficacy and safety of tenofovir didoproxil fumarate (TDF) in asians with HBeAg-positive and HBeAg-negative chronic hepatitis B (CHB), preliminary analysis. Hepatology. 2010;52:559A. [Cited in This Article: ] |
| 93. | Marcellin P, Buti M, Gane EJ, Krastev Z, Flisiak R, Germanidis G, Washington KM, Barnes CN, Flaherty JF, Bornstein JD. Five years of Treatment with Tenofovir DF for Chronic Hepatitis B Infection is Associated with Sustained Viral Suppression and Significant Regression of Histological Fibrosis and Cirrhosis. 62th Annual Meeting of the American. Association for the Study of Liver Diseases. 2011;Nov 6-9; San Francisco. [Cited in This Article: ] |
| 94. | Afdhal N, Buti M, Fung S, Gane E, Faherty J, Martins E, Bekele N, Bornstein J, Marcellin P. Factors associated with regression of cirrhosis in patients with chronic hepatitis B infection treated with tenofovir disoproxil fumarate. J Hepatol. 2012;56:S196. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 95. | Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, Lee SS, Chan S, Shiffman ML, Washington MK. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch Intern Med. 2006;166:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 96. | Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 97. | Berg T, Marcellin P, Moller B, Zoulim F, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D. Tenofovir disoproxil fumarate (TDF) versus emtricitabine plus TDF (FTC/TDF) for treatment of chronic hepatitis B (CHB) in patients with persistant viral replication recieving adefovir dipivoxil: final week 168 results. Hepatology. 2010;52:95A. [Cited in This Article: ] |
| 98. | Piao CY, Fujioka S, Iwasaki Y, Fujio K, Kaneyoshi T, Araki Y, Hashimoto K, Senoh T, Terada R, Nishida T. Lamivudine treatment in patients with HBV-related hepatocellular carcinoma--using an untreated, matched control cohort. Acta Med Okayama. 2005;59:217-224. [PubMed] [Cited in This Article: ] |
| 99. | Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 100. | Kubo S, Tanaka H, Takemura S, Yamamoto S, Hai S, Ichikawa T, Kodai S, Shinkawa H, Sakaguchi H, Tamori A. Effects of lamivudine on outcome after liver resection for hepatocellular carcinoma in patients with active replication of hepatitis B virus. Hepatol Res. 2007;37:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Kim BK, Park JY, Kim do Y, Kim JK, Kim KS, Choi JS, Moon BS, Han KH, Chon CY, Moon YM. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28:393-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 102. | Hann HW, Bergin D, Coben R, DiMarino AJ. Prevention of new hepatocellular carcinoma with concomitant antiviral therapy in chronic hepatitis B patients whose initial tumor was successfully ablated. Int J Cancer. 2011;128:739-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 657] [Cited by in F6Publishing: 665] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 105. | Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54:12-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 106. | Hongthanakorn C, Chotiyaputta W, Oberhelman K, Fontana RJ, Marrero JA, Licari T, Lok AS. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. Hepatology. 2011;53:1854-1863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 107. | Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, Xie R, Ge S, Hu J, Jiang Z. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment Pharmacol Ther. 2011;34:344-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Gill US, Al-Shamma S, Burke K, Ross V, Marley R, Kooner P, Foster GR, Kennedy PTF. Factors determining bone mineral density loss in chronic hepatitis B patients: is tenofovir disoproxil fumarate the main culprit? Gut. 2011;60:A230. [DOI] [Cited in This Article: ] |
| 109. | Murray KF, Szenborn L, Wysocki J, Rossi S, Corsa AC, Dinh P, McHutchison J, Pang PS, Luminos LM, Pawlowska M. Randomized, placebo-controlled trial of tenofovir disoproxil fumarate in adolescents with chronic hepatitis B. Hepatology. 2012;56:2018-2026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 110. | Kim YJ, Cho HC, Sinn DH, Gwak GY, Choi MS, Koh KC, Paik SW, Yoo BC, Lee JH. Frequency and risk factors of renal impairment during long-term adefovir dipivoxil treatment in chronic hepatitis B patients. J Gastroenterol Hepatol. 2012;27:306-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10:941-96; quiz e68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 112. | Kim WR, Benson JT, Hindman A, Brosgart C, Fortner-Burton C. Decline in the need for liver transplantation for end stage liver disease secondary to hepatitis B in the US. Hepatology. 2007;46:238A. [Cited in This Article: ] |